Current Research
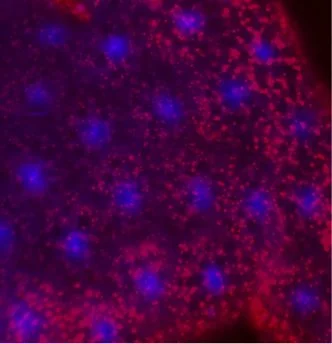
Lamp1 mutants have an acidified endolysosomal system.
Lysosomal Acidification:
Increasing disease risk with age shortens healthspan and causes a significant economic burden for our society. In particular, neurodegenerative diseases (NDs) including dementias are detrimental for the older population in part due to a decline in proteostasis over time. The most frequent NDs, Alzheimer’s disease (AD) and Parkinson’s disease (PD) affect more than 10% of elderly Americans.
Accumulating evidence suggests a critical link between NDs and the pH of lysosomes, the organelles responsible for the degradation and recycling of many macromolecules. For example, several AD associated mutations cause an elevated lysosomal pH concomitant with impaired proteolysis. PD risk factors can affect lysosomal pH as well, with Glucocerebrosidase 1 (GCase, encoded by GBA1) mutations lowering the pH of the endolysosomal system, indicating that pH alterations in either direction can be detrimental.
In collaboration with the lab of Dr. Gustavo MacIntosh at Iowa State University, we have recently shown that, similar to Gba1, mutation in Drosophila lysosome associated protein Lamp1 causes excessive endolysosomal acidification (EELA) in the fat bodies (similar to the mammalian liver and adipose tissue). Indeed, we identified additional similarities between the loss of Gba1 and Lamp1 including lipid defects, the accumulation of protein aggregates in brains, and neuroinflammation. Our current research focuses whether and how Lamp1 and Gba1 interact to control lysosomal pH and neurodegeneration, as mutation of Lamp1 increases the frailty of flies expressing a pathogenic form of α-Synuclein, a protein often responsible for Parkinson.
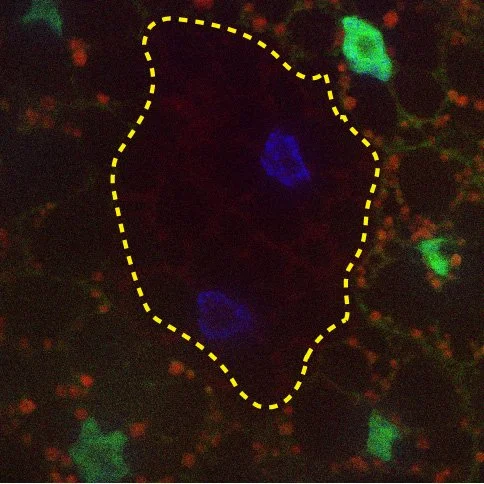
eMI is ESCRT machinery dependent
e-MI:
Proper turnover of proteins and organelles is essential for normal cell function. Global decline in the efficiency of cellular quality control systems leads to liver and neurodegenerative diseases e.g. due to protein aggregation, in particular at old age. Damaged or altered cytosolic proteins are cleared by the proteasome and autophagy. Importantly, autophagy also provides nutrients including amino acids and lipids to cells under stress and is thus essential for energy balance.
There is a strong correlation between lifespan extension, age related diseases and levels of autophagy. Not surprisingly, autophagy declines with age and is associated with the accumulation of altered, defective components in all model organisms examined, including mice and Drosophila, and experimental reduction of autophagy in animal models leads to reduced organ function and a shortened life span.
Although Macroautophagy (MA) can selectively degrade organelles or aggregated proteins, selective degradation of single proteins has only been described for Chaperone Mediated Autophagy (CMA) and endosomal Microautophagy (e-MI). CMA is specific for proteins containing a KFERQ-related targeting motif. e-MI, the uptake of proteins into multivesicular bodies via the ESCRT machinery can occur in bulk and in a KFERQ dependent manner. In collaboration with the Cuervo lab in our department, we have identified eMI in Drosophila melanogaster. We are currently using the power of genetics to identify eMI regulators and to define its physiological role.
Because altered autophagy contributes to liver, kidney, and neurodegenerative diseases, our research will significantly advance our understanding of how dysfunction of eMI may cause disease, ultimately leading to the development of novel strategies for treatments.
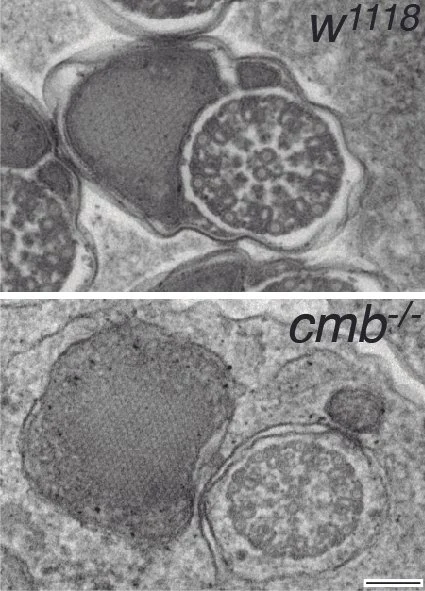
Spermatid individualization defects in Combover mutants
Fertility:
Spermatogenesis, Rho Kinase and the Intrinsically Disordered Protein Combover
About ten percent of couples have fertility problems, many of which are caused by defective spermatogenesis. The development of gametes depends on genetic programs that control their differentiation and morphogenesis. It requires the coordination of cell division, polarity, movement, and shape. Sperm initially develop as syncytia that then differentiate and individualize, processes that rely on the coordinated interplay between the actin and microtubule cytoskeletons with the overlaying plasma membrane.
Rho kinase (Rok/ROCK) is known to coordinate cellular signaling with cell shape and migration in somatic cell migration and its misregulation causes severe developmental defects and promotes metastasis formation. We have discovered a previously unknown and unanticipated function of Rok in the male germline, where it is required for spermatid individualization and its lack causes infertility. This phenotype is very similar to the loss of the Rok substrate Combover (Cmb), a member of the growing family of intrinsically disordered proteins (IDPs), and, indeed, Cmb function in spermatogenesis requires intact Rok phosphorylation sites. Intriguingly, Cmb interacts with the axonemal component Rsp3, and members of a conserved protein network including the E3-ligase Mind bomb 2 and the membrane raft proteins Flotillin 1/2, each of which affects spermatid development. Our current research addresses the question how Rok and Cmb coordinate sperm tail formation with individualization, potentially integrating the growth of the axoneme with membrane encapsulation, a process poorly understood in any organism.
Our research may have wide-ranging implications for sperm development and male fertility beyond Drosophila: haploid mammalian spermatids also develop as ‘functionally diploid’ syncytia and individualize during spermiation. In mammals and flies this occurs in a coordinated fashion after the growth of the axoneme, and requires tight encapsulation of the sperm tail in its membrane while shedding unnecessary cytoplasm. As in flies, aberrant individualization causes sterility in men.